כיצד לבצע הדברת יונים
הדברה כימית של מזיקים שונים, היא הדברה אשר לרוב מתבצעת בעזרת חומרים כימיים שמטרתם להרוג את המזיקים השונים לצורך הפינוי
בניית אתר אינטרנט כחלק חיוני מצמיחת העסק
החלטתם לפתוח עסק חדש ואפילו בחרתם שם מקורי וקליט? אחרי שביצעתם את רישום החברה, חשוב להקדיש משאבים כמו זמן וכסף
לוכד חולדות
אנשים רבים נאלצים להתמודד עם תופעה לא נעימה של חולדות או עכברים המתחפרים להם במחילות בין קירות הבית שלהם. אלה

בחירת העורך
עוסק מורשה סטודנט
סטודנטים רבים מקימים את העסק כבר לפני או במהלך התואר, לרבות אלה שמיעדים לעצמם להיות עצמאיים. אותם סטודנטים מתחילים כעוסק
מרחיק יונים מקצועי
מרחיק יונים – אומרים ליונים שלום ולא להתראות! היונים מקננות במסתור הכביסה, מלכלכות את האזור ולעתים גם את הכביסה
עזרה ראשונה שיניים
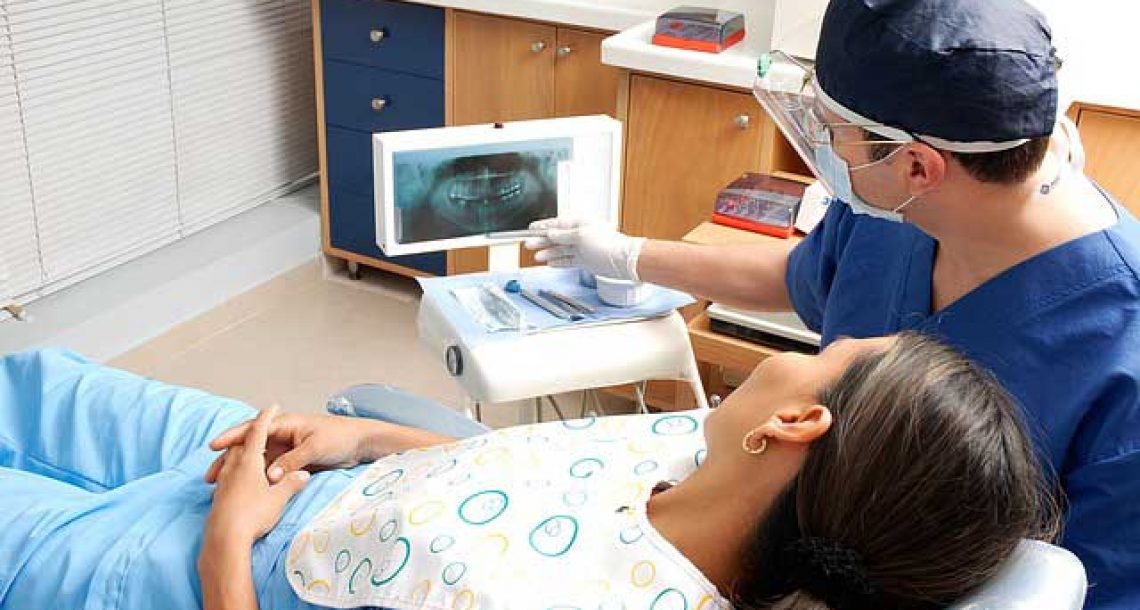
הקדמה דברים מופתיים קורים לנו ולכולם במהלך היום. אי אפשר לחזות כאב, אי אפשר לחזות מה יהיה בהמשך היום, או

הכי פופולארי
מומחה להשתלות שיניים
הקדמה הרפואה התקדמה במהלך השנים בצורה דרסטית מאוד. ממצב שבו אנשים היו מתים כל יום בגלל רפואה כושלת, עד
שמאי מקרקעין בגבעתיים
איך לבחור רופא שיניים טוב בירושלים?
קידום אתרים
חובותיו של עוסק זעיר מס הכנסה
כל עסק אשר מוגדר כעוסק זעיר הוא עסק בעל חובת דיווח שנתית למס הכנסה ולמע"מ בנוגע להיקף הכנסותיו השנתיות וכן
רופא שיניים בירושלים
עזרה ראשונה שיניים
מומחה פה ולסת
סיפורים נוספים
הגשת דוח שנתי – מה זה כולל
כאשר אדם מקים עסק אשר מוגדר כעוסק פטור, משמעות הדבר היא שהעסק של האדם לא חייב בתשלום למס הכנסה או
מומחה לשיקום הפה
מה זה מומחה לשיקום הפה? מומחה בשיקום הפה, או בשמו הלועזי פרוסטודונט, הוא רופא שיניים אשר מתמחה באסתטיקה, שיקום, והחלפת
עוסק מורשה
עוסק מורשה –או חברה בע"מ – מה עדיף? בשנים עברו כאשר אדם רצה להקים חברה בע"מ היה עליו לחבור לשותף